
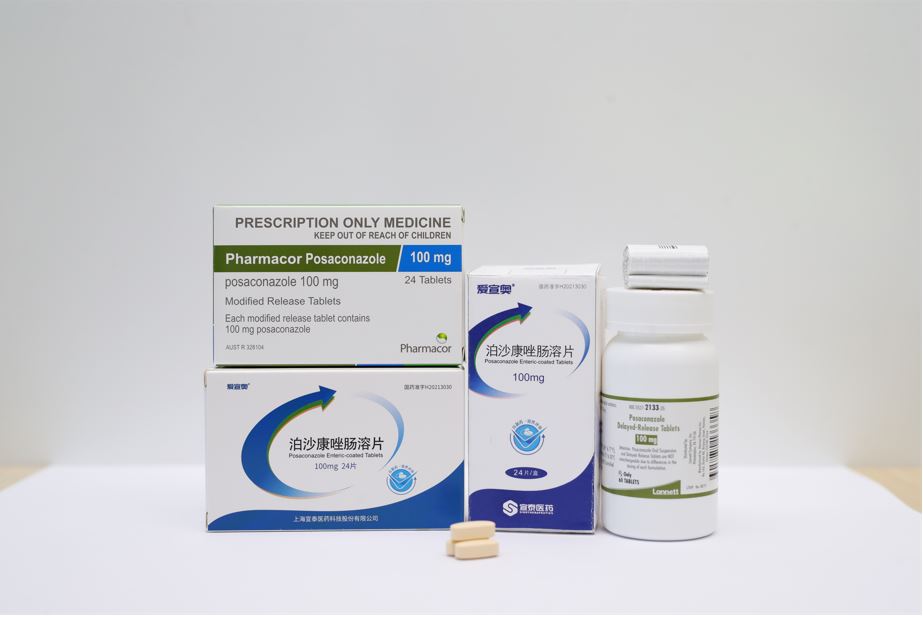
Recently, SinoT’s generic drug Posaconazole delayed-release tablets (100mg) has been granted approval from the Health Sciences Authority (HSA) in Singapore.
Up to now, the product has been marketed in China, the United States and Australia. The approval of the product in Singapore will bring a positive impact on company’s business growth overseas.
About SinoT
SinoT is a specialty pharma with focus on the research & development, commercialization and sale of complex generics and 505(b2) products. We dedicated to developing high quality medicines that meet with international standards. We’ll do more than our best to benefit the patients in the future.
About PosaconazolePOSACONAZOLE is a member of the group of triazole antifungals,which is used to prevent certain kinds of fungal or yeast infections. With the increasing number of high-risk patients such as cancer chemotherapy, transplantation, HIV/AIDS infection, and diabetes, the overall incidence of invasive fungal infections (IFIs) continues to rise. Different from other drugs used to treat IFIs, posaconazole can be used to prevent IFIs , so it has a broad market prospect.
Shanghai Head Office

99 Haike Road, Bldg. 3, 1st Flr., Pudong District Shanghai 201210, P.R. China
Manufacturing Site in Jiangsu

No. 163 Zhuhai Road, Haimen Area, Nantong City, Jiangsu Province
